March 29, 2023
Feed Them or Lose Them
How developing nerve cells are influenced by essential amino acids
Our developing brains demand the right nutrients at the right times. This sustenance provides essential energy for cellular processes that underlie brain formation. But what happens if these substances aren’t available? Professor Gaia Novarino’s group at the Institute of Science and Technology Austria (ISTA) has shown that a shortage of essential amino acids results in severe developmental problems in mice and humans, causing lasting effects in life.
Brain development consists of a sequence of coordinated steps, which are mainly instructed by our genes. During these steps, the proper positioning and functionality of nerve cells in the brain (neurons) are critical—nonfunctional or incorrectly positioned neurons can lead to severe neuropathological consequences. Mutations in genes coordinating this program are often linked to neurodevelopmental disorders; however, environmental stressors such as nutrient scarcity or malnutrition can also influence the development of the brain. Still, very little is known about the importance of specific nutrients and the role of metabolism during brain development.
Professor Gaia Novarino and her team at ISTA have now shed light on this brain mystery. In collaboration with several Viennese universities, the scientists profiled the nutritional program of the mouse brain. They found that a group of amino acids—the building blocks of proteins—play a key role during certain stages of brain development. Starving nerve cells of these amino acids led to severe effects after birth. Mice developed microcephaly—a reduction in brain size—that persisted into adulthood, eventually causing long-term behavioral changes similar to the ones observed in autism spectrum disorders (ASD). The findings were published today in Cell.
Tracing nutrients
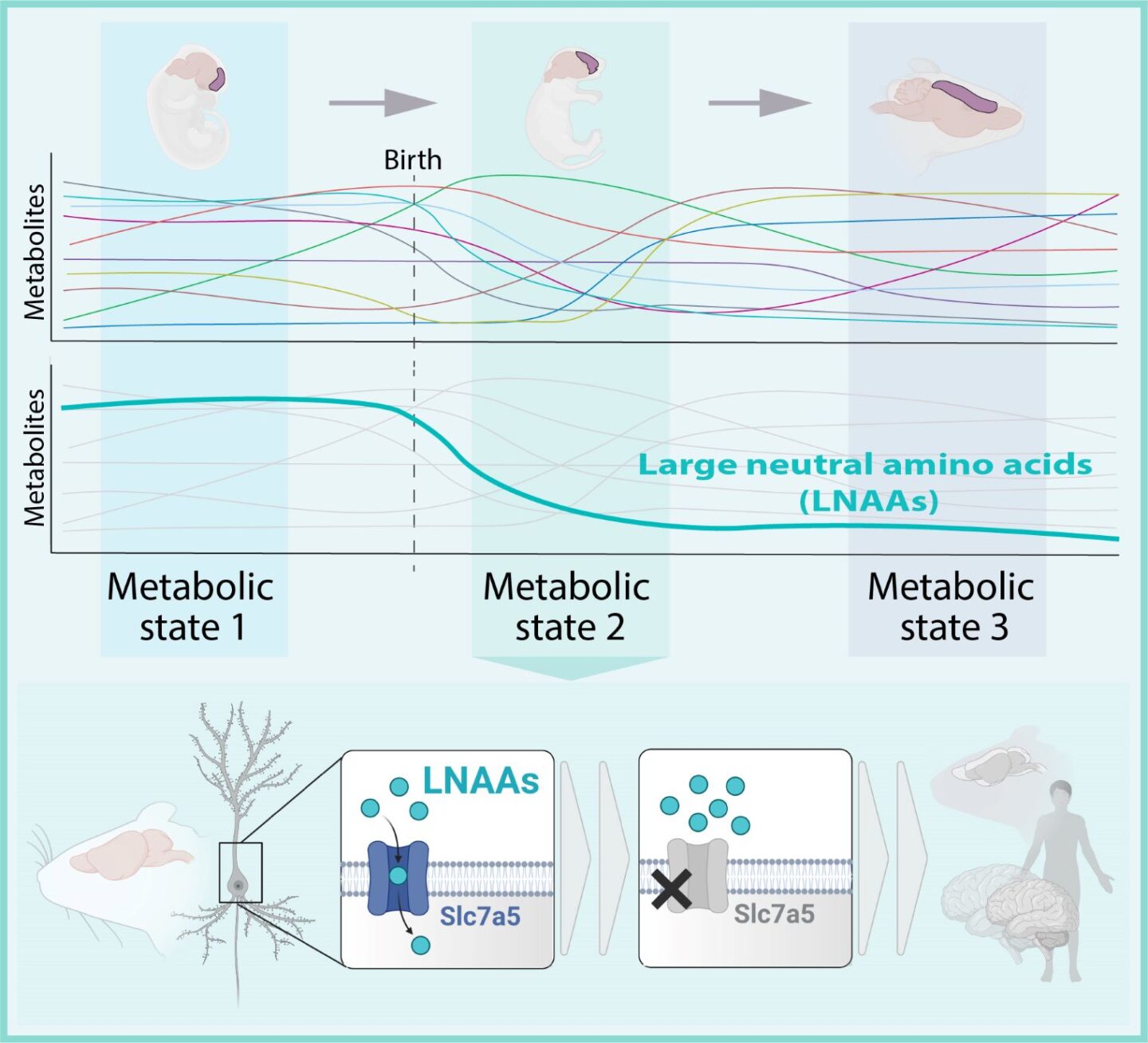
Metabolites are substances made or used when we break down food, thus fueling our bodies. One set of these metabolites—large neutral amino acids (LNAAs)—caught the scientists’ eyes. LNAAs are essential amino acids that the body cannot synthesize on its own and must be taken up via food. “By checking the levels of metabolites throughout brain development, they seemed especially important for the neurodevelopmental period after birth,” explains first author and doctoral student Lisa Knaus. Previously, the Novarino group identified a novel form of autism where patients could not transfer LNAAs into the brain due to a genetic defect in a gene labeled SLC7A5. This possible link triggered the young scientist’s investigative nature. “We got really interested in the amino acids’ role in brain development.”

Starvation leads to smaller brains
The researchers proceeded by performing a conditional knockout experiment—an approach in which a specific gene is deleted in certain mouse cells, giving rise to the mutant mouse strain. The strains are then compared to healthy mice, allowing scientists to evaluate if the depletion leads to a change in characteristic traits.
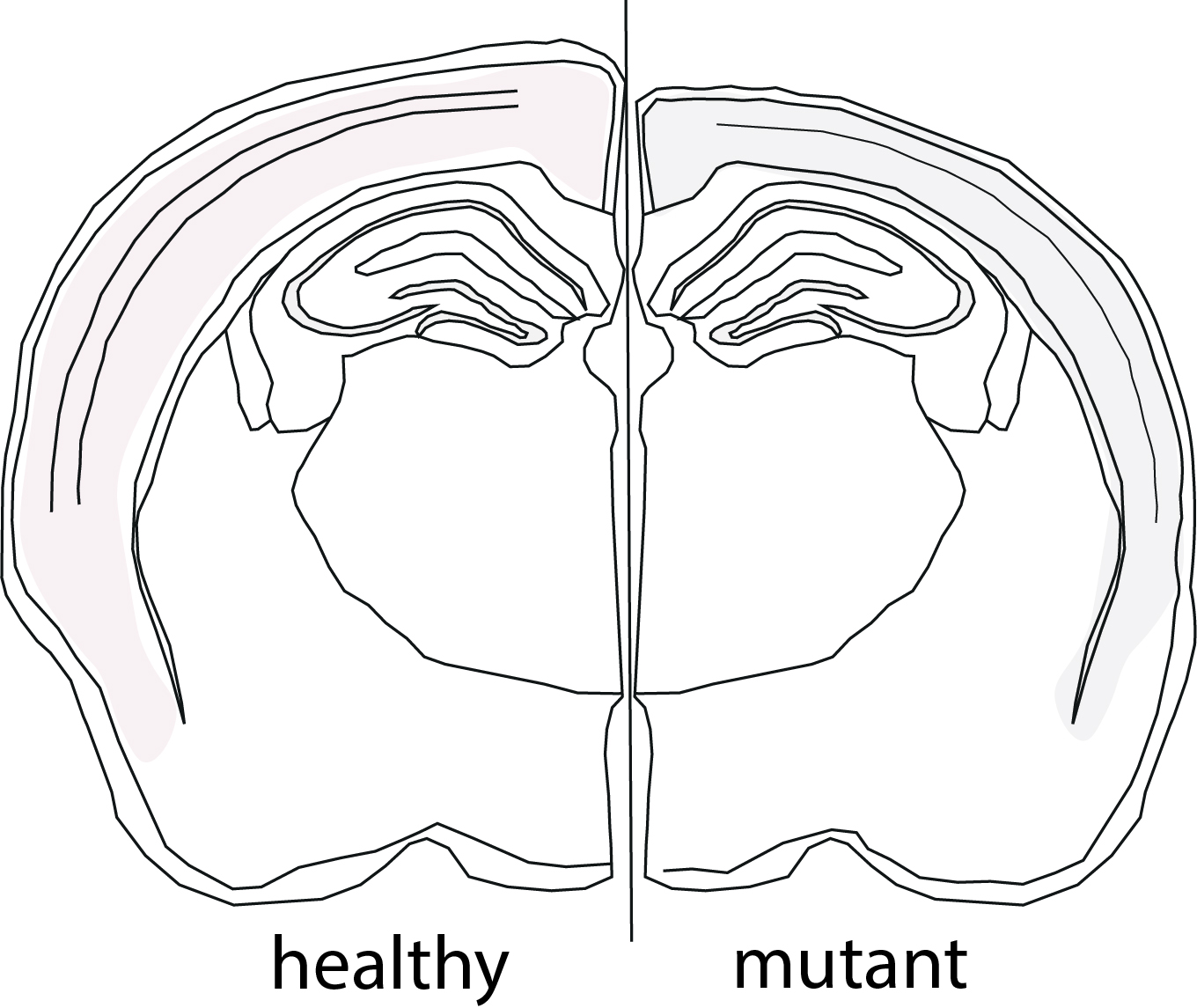
In this case, the group deleted the Slc7a5 gene, which carries the instructions to build the transporter that brings LNAAs into the nerve cells. In other words: the neurons were starved of essential amino acids. In the embryo stages, brain formation appeared to be fine. However, right after birth, nerve cells started to be affected by the low levels of LNAAs. In this period, mutant mice developed microcephaly due to a reduction in the thickness of the cortex—the outer layer of the brain—when compared to healthy mice.
Death of neurons
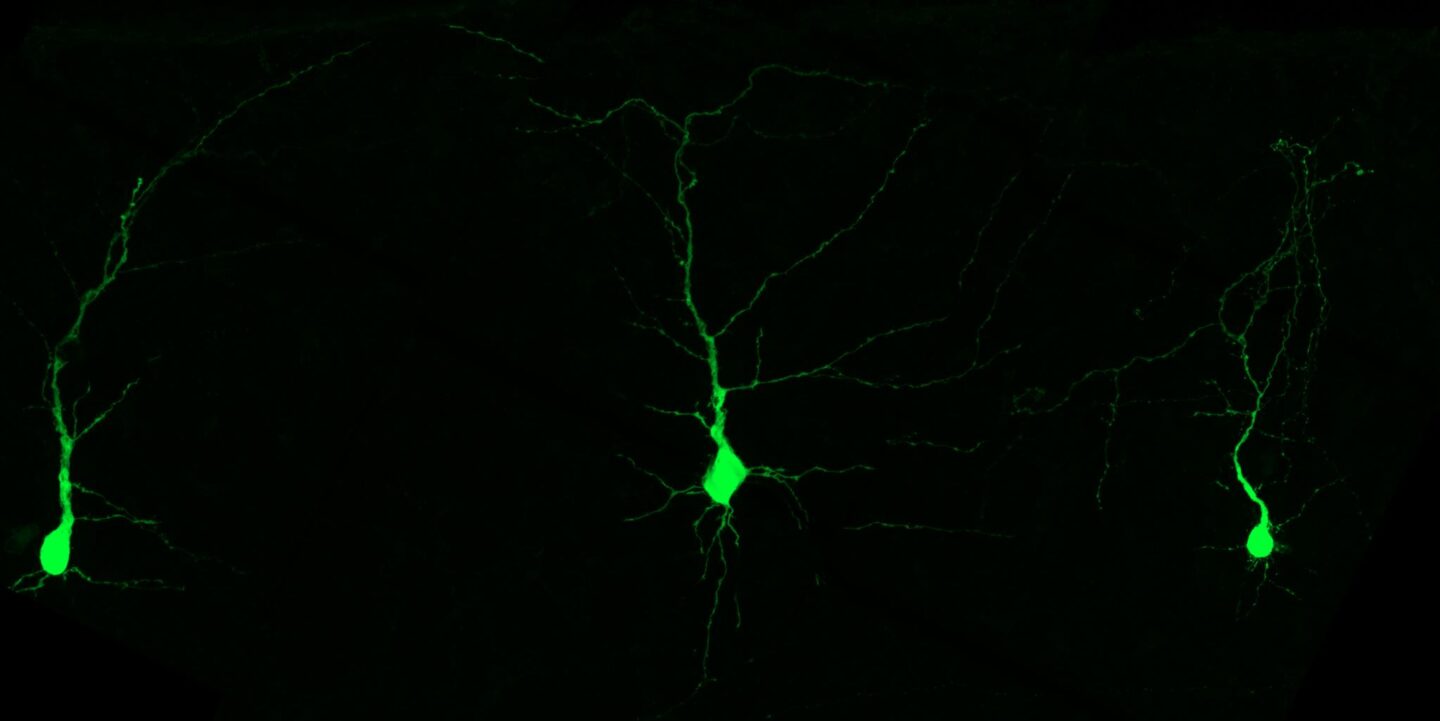
Eager to understand more, the scientists employed a method to label and manipulate individual neurons. They found that a large fraction of neurons in the upper layer of the cortex vanished during the first days after birth. The cells were dying—but why? It turns out that neurons lacking LNAAs are less active. “Neurons that aren’t firing properly get eliminated shortly after birth. It’s like natural selection, where only the fittest cells survive,” Knaus explains.
Long-term changes in behavior
Neuron death and activity rates normalized after the critical period. Nevertheless, the smaller brain size persisted until adulthood. Mutant mice started to show several behavioral abnormalities, including motor deficits, sociability defects, and hyperactivity. Though not a complete representation, these behavioral patterns are very similar to the ones in patients with mutations in the SLC7A5 gene, who also exhibit microcephaly, autism, and motor deficits.
Knaus concludes: “Our work presents a detailed look at how even small changes in the metabolism and nutrient availability can have severe consequences for brain development and function.”
Publication:
L. S. Knaus, B. Basilico, D. Malzl, M. Gerykova Bujalkova, M. Smogavec, L. A. Schwarz, S. Gorkiewicz, N. Amberg, F.M. Pauler, C. Knittl-Frank, M. Tassinari, N. Maulide, T. Rülicke, J. Menche, S. Hippenmeyer & G. Novarino. 2023. Large neutral amino acid levels tune perinatal neuronal excitability and survival. Cell. DOI: 10.1016/j.cell.2023.02.037
Funding information:
This work was supported by the Austrian Science Fund (FWF, DK W1232-B24) and by the European Union’s Horizon 2020 research and innovation program (ERC) grant 725780 (LinPro) to S. Hippenmeyer and 715508 (REVERSEAUTISM) to G. Novarino.
Information on human subjects and animal studies:
All animal protocols were approved by the Institutional Animal Care and Use Committee at ISTA and by the Bundesministerium für Bildung, Wissenschaft und Forschung, Austria (approval number: BMBWF-66.018/0015-V/3b/2019). For the human participants (patients and their parents), written informed consent, and collection of data and samples were obtained according to a protocol approved by the Ethics Committee of the Medical University of Vienna (protocol number 1443/2020).



