September 22, 2016
First complete architecture of respiratory supercomplexes in mammalian mitochondria determined by IST Austria Professor Leonid Sazanov and his group
Different assembly stages of supercomplexes identified with cryo-electron microscopy | Results published in Nature
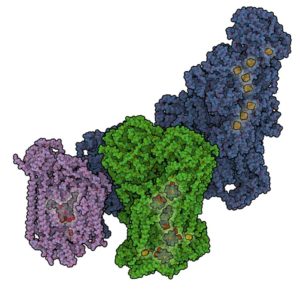
The respiratory chain consisting of several large protein assemblies, which are embedded in the mitochondrial lipid membrane, takes care of the energy production in human cells. These large electron transport chain complexes are organized in so-called supercomplexes (respirasomes). IST Austria Professor Leonid Sazanov and his group were now able to determine the structure of such mitochondrial supercomplexes in their physiologically relevant state in mammalian (ovine) cells for the first time. They showed that the respirasome exists in two major forms – a ‘tight’ and a ‘loose’ form. These observations lead to the understanding of supercomplex assembly and disassembly as the forms likely represent different stages of this process.
Leonid Sazanov and his group members recently solved the first full atomic structure of the largest component of the respiratory chain – complex I, which was also published in Nature a few weeks ago. However, in vivo in the mitochondrial membrane respiratory complexes do not exist in isolation but are organized in huge “supercomplexes” (up to ~ 2 Megadalton in size). The reason was unclear up to date. Sazanov’s previous findings and specific methods developed now enabled the researchers to determine the architecture of the physiological supercomplexes as well. They analysed several types of these supercomplexes using recently developed cryo-electron microscopy methods and described the arrangement and environment of all active sites in the component enzymes (complexes I, III and IV). It appears that apart from the stabilization of the individual protein assemblies, one of the functions of supercomplexes may be to prevent excessive production of oxygen radicals. Such radicals are harmful to the DNA and proteins in the cell, and could represent one of the causes of aging. In addition, the insight into the architecture of the mitochondrial supercomplexes will serve as a starting point for the understanding of diseases connected to dysfunctions of the respiratory chain.
Leonid Sazanov, a Belarusian-British structural biologist, studied biophysics (B.Sc. and M.Sc.) at the Belarusian State University in Minsk and performed his doctoral studies at the Department of Biophysics at the Moscow State University where he remained as a research fellow in the group of Sergei V. Zaitsev. After continuing his research in groups at the University of Birmingham and at Imperial College in London, Sazanov joined the group of Nobel Laureate John E. Walker at the MRC Laboratory of Molecular Biology in Cambridge as a research associate. After his position as tenured program leader at the MRC Mitochondrial Biology Unit in Cambridge Leonid Sazanov joined IST Austria in April 2015 as Professor. The research group aims to understand the structure and function of membrane proteins and focuses on the determination of the structure and mechanism of respiratory complex I.



