November 2, 2022
Whole Tissue Shapes Brain Development
Mechanism of neuron production uncovered – Study in Science Advances
The brain’s neocortex is what makes us humans uniquely us. How it develops is, however, not yet fully understood. Errors in the process lead to diseases such as microcephaly – ‘small brain’ – and macrocephaly – ‘big brain’. Simon Hippenmeyer’s group at the Institute of Science and Technology Austria has now explained a tissue-wide mechanism controlling neuron production from stem cells. This is the result of a genetics study on mice, published today in Science Advances.
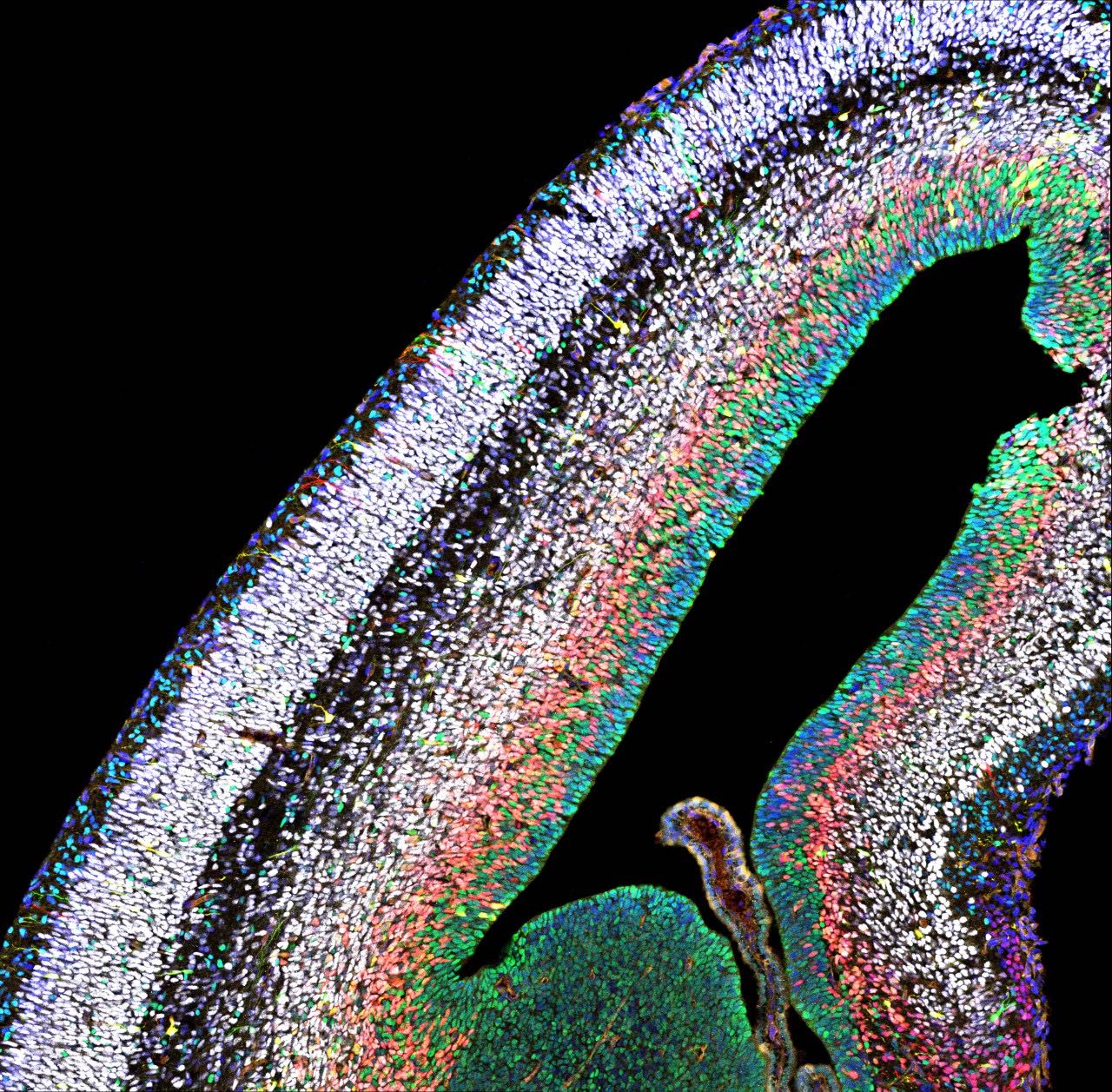
In humans, the neocortex is the seat of cognition, allowing us to think, imagine and dream. During embryonic development, a big pool of stem cells gives rise to a kaleidoscope of neurons and glia cells- non-neuronal cells in the brain. However, this intricate process is error-prone: Mistakes can cause too few or too many neurons to be produced, leading to diseases like micro- or macrocephaly.
Color-coded neurons hold the cue
Simon Hippenmeyer and his group at the Institute of Science and Technology Austria (ISTA) sought to understand the mechanisms that control how stem cells generate neurons and glia, both at the individual stem cell level and in the holistic context of the whole tissue. To do so, they made use of the MADM technique established by Hippenmeyer. With MADM, the scientists can color-code cells in the brain, while at the same time removing particular genes implicated in brain development either simply in the single cell or globally in the entire brain. “This allows us to visualize individual cells and see how they react when certain genes are switched off, either in the whole tissue or just in the single cell”, explains Nicole Amberg, former Postdoc in the Hippenmeyer group at ISTA.
The neuroscientists particularly focused on the role of PRC2. PRC2 is a so-called epigenetic regulator, which means that PRC2 controls the degree of DNA packaging. When this protein complex is lost, mice show dramatic microcephaly, with a neocortex that is only half as large as the cortex of normal mice. How PRC2 regulates the production of neurons was so far unknown. However, as epigenetic complexes usually act on the DNA in the nucleus, it was extrapolated that PRC2 most likely acts intrinsically and only within each single stem cell. Hippenmeyer and Amberg decided to test this idea. “With MADM, we can trace whether stem cells produce the correct types of neurons, in the correct numbers, both when PRC2 is lost only in a single stem cell and compare to conditions when it is lost in the entire tissue”, says Amberg.
When the researchers removed PRC2 from just a single stem cell, the mice’s neocortex remained unchanged. “The stem cell doesn’t really care and produces a normal amount of neurons. When PRC2 is lost from a single stem cell, the neuronal part of the neocortex is not altered at all: We see the same number of neurons, same subtypes, in the correct ratio”, recalls Amberg. This observation was puzzling, as knocking out PRC2 in the whole tissue causes only half the neurons to be formed. “So where does the problem lie? These findings point to a mechanism that acts on the whole-tissue level.”
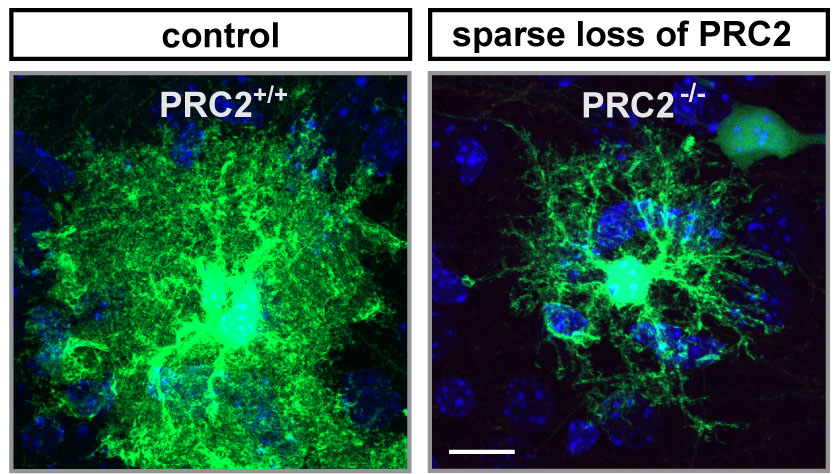
Neighboring cells affect stem cell development
The researchers then dug deeper and looked at gene activity. Indeed, they found dramatic differences in which genes were active and which ones were not, depending on whether they had removed PRC2 in a single stem cell or the whole tissue. Removing PRC2 from the whole developing neocortex caused gene expression to be deregulated also in neighboring cells, including the stem cells. This suggests that losing PRC2 in the stem cell niche affects how the stem cell develops. “We didn’t anticipate such a widespread role for PRC2. It is surprising that neighboring cells matter so much to the stem cell – especially as PRC2 does not code for a receptor or a signal, but acts within the cell’s nucleus”, says Hippenmeyer.
Indeed, how losing PRC2 in the entire tissue affects stem cell development mechanistically is not clear yet. Further findings showed that loss of PRC2 leads to waves of gene deregulation. “Knocking out the PRC2 complex changes gene expression, this then creates a secondary wave of deregulation – and the genes in this second wave matter for the microcephaly phenotype”, explains Amberg.
While PRC2 loss in a single stem cell doesn’t affect the development of neurons, this loss does affect astrocyte – a critical glia cell-type – development: Typically, after a stem cell has given rise to neuronal progenitors, it then gives rise to astrocyte progenitors. When PRC2 is lost in the stem cell, however, these astrocytes do not develop properly.
While the study was conducted in mice, the findings have relevance also for human development, says Hippenmeyer. “A global gene knockout, switching off a gene in the entire tissue, may actually have more severe effects on development. Also, having an inherited mutation that affects the whole tissue may lead to a worse outcome than acquiring a mutation later in embryonic life when it only affects fewer cells.”
Publication:
Amberg et al. 2022. Tissue-wide Genetic and Cellular Landscape Shapes the Execution of Sequential PRC2 Functions in Neural Stem Cell Lineage Progression. Science Advances.
DOI: 10.1126/sciadv.abq1263
http://www.science.org/doi/10.1126/sciadv.abq1263
Funding information:
The first author received funding from the FWF Firnberg-Programm (T 1031). The work was supported by ISTA institutional funds and by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 725780 LinPro) to Simon Hippenmeyer.



