August 20, 2024
Re-engineering Bacterial Defense Systems
New ISTA Assistant Professor Jack Bravo develops potent tools for genome engineering
The Nobel Prize-winning technology CRISPR-Cas9 is one of multiple biotech breakthroughs in gene editing based on re-engineered bacterial immune system enzymes. However, new genome engineering tools could still be hidden in the haystack of unexplored microbial defense mechanisms. This July, the Institute of Science and Technology Austria (ISTA) welcomed Assistant Professor Jack Bravo who seeks to shape the future of biotech advancements. His work could find applications in re-sensitizing superbugs to antimicrobial therapy.

You started your PhD in 2015, shortly after the development of CRISPR-Cas9 as an unprecedented genome-editing tool. This technology made it possible to cut and insert specific genes in living cells. Did that motivate you to join this field?
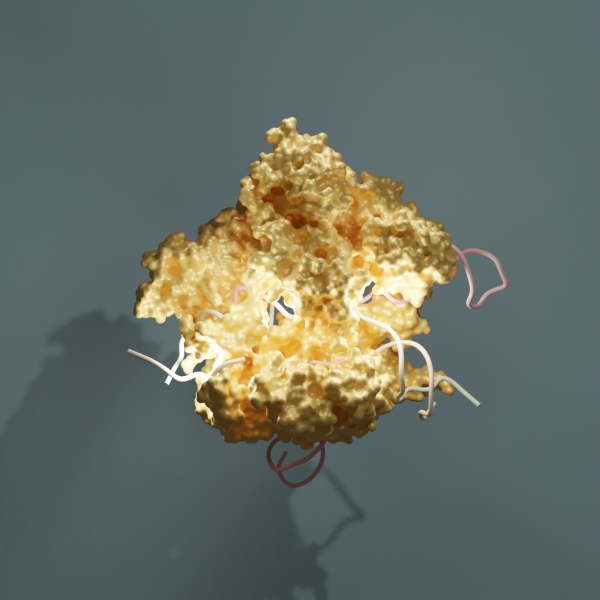
I was indeed aware of the exciting developments with CRISPR-Cas9, but I only started working on this topic and how to optimize microbial defense mechanisms after I defended my PhD in 2019. Until then, I studied virus assemblies using structural biology and biophysics techniques. I took the lockdowns during the COVID-19 pandemic as a chance to dive into the overwhelming literature on CRISPR-Cas9 and do lots of background reading. I also wanted to specialize in cryo-electron microscopy, a fast-advancing molecular imaging technique. With cryo-EM, biological samples can be observed in their natural state and at near-atomic scales. This has led me to join David Taylor’s lab as a postdoc at The University of Texas at Austin. This career opportunity allowed me to tackle both subjects at once, bacterial immune systems and cryo-EM.
“We are starting to grasp that bacteria have a plethora of enigmatic defense mechanisms that require exquisite specificity.”
Having yourself patented three re-engineered and optimized CRISPR-Cas9-related enzymes, how do you envision the next biotech breakthrough in genome engineering?
Over 150 bacterial immune systems have been described in the last six years and there is still much untapped potential in this area. We are starting to grasp that bacteria have a plethora of enigmatic defense mechanisms that require exquisite specificity. These defense mechanisms are fantastic tools in molecular biology and I am really keen on exploring diverse systems, dissecting how they work, and figuring out how to optimize them for genome editing purposes.
If you were a bacterium, what would your ideal immune defense system look like?
One less explored mechanism is how bacteria defend themselves from plasmids. Plasmids are small pieces of circular DNA that can move between different bacteria and strains. Plasmids function like parasites as they reduce the reproductive rate of the host bacterium. Also, their evolutionary fate is not linked to a specific bacterial strain. They bring in new genes that could benefit the bacteria, like antibiotic resistance genes, but their presence is a burden on the cells. Thus, bacteria usually want to get rid of the plasmids, while at the same time trying to keep the beneficial genes.
Speaking of antibiotic resistance, the other side of the coin of bacterial immune systems is when patients are infected with ‘superbugs.’ This was described as the ‘silent pandemic.’ Are you looking for a next-generation ‘super antibiotic’?
An emerging treatment against antibiotic-resistant bacteria is phage therapy. It consists of using special viruses called “bacteriophages,” which can be translated as “bacteria-devouring”. Several strong antibiotics have a broad spectrum, meaning they can target various bacterial strains, but also wipe out the patients’ microbiomes–such as the gut flora. On the other hand, bacteriophages are very selective in the strains of bacteria they are effective against. Thus, phage therapy can be used to target a specific spectrum of pathogenic bacteria, either alone or in conjunction with antibiotics. However, pathogenic bacteria can also evolve resistance against phages. It is a fine balancing act. My work could find an application in uncovering ways to re-sensitize bacteria to phage therapy.

“I seek to re-engineer the ‘molecular blueprints’ of bacterial defense systems to help make them more efficient and specific.”
Are you motivated by finding new therapies against specific diseases?
I am mainly interested in understanding the molecular mechanisms underlying bacterial immune systems and how to optimize them. I seek to re-engineer the ‘molecular blueprints’ of bacterial defense systems to help make them more efficient and specific.
Your career led you from the University of Leeds through The University of Texas at Austin to ISTA. How would you describe your journey?
As a structural biologist in training, I sought to develop further in a lab more experienced in cryo-EM, a technique that has boomed in studying protein complexes in the last few years. This led me to first move to The University of Texas at Austin. I then chose ISTA because of its recognized strength in cryo-EM, but also because of how diverse and interdisciplinary it is. Unlike other academic research institutions where departments are segregated, ISTA makes it very easy to bounce ideas and collaborate with scientists in various areas of expertise. I am glad to already have two lab members, a lab manager and a postdoc, who joined me from my first month here. I look forward to seeing how my team will develop and am excited to connect with fellow researchers with diverse scientific backgrounds.



