February 5, 2024
Down to the Core of Poxviruses
ISTA researchers uncover the architecture of poxvirus cores
A recent re-emergence and outbreak of Mpox brought poxviruses back as a public health threat, underlining an important knowledge gap at their core. Now, a team of researchers from the Institute of Science and Technology Austria (ISTA) lifted the mysteries of poxviral core architecture by combining various cryo-electron microscopy techniques with molecular modeling. The findings, published in Nature Structural & Molecular Biology, could facilitate future research on therapeutics targeting the poxvirus core.

Variola virus, the most notorious poxvirus and one of the deadliest viruses to have afflicted humans, wreaked havoc by causing smallpox until it was eradicated in 1980. The eradication succeeded thanks to an extensive vaccination campaign using another poxvirus, the aptly named Vaccinia virus. The 2022-2023 re-emergence and outbreak of Mpox virus reminded us once more that viruses find ways to return to the forefront as public health threats. Importantly, this has highlighted the fundamental questions about poxviruses that have remained unanswered to this day.
One such fundamental question lies, quite literally, at the core of the matter: “We know that for poxviruses to be infective, their viral core must be properly formed. But what is this poxviral core made of, and how do its individual components come together and function?” asks ISTA Assistant Professor Florian Schur, the corresponding author of the study. Schur and his team now put their finger on the missing link: a protein called A10. Interestingly, A10 is common to all clinically relevant poxviruses. In addition, the researchers found that A10 acts as one of the main building blocks of the poxviral core. This knowledge could be instrumental for future research on therapeutics targeting the poxviral core.

“The most advanced cryo-EM techniques available today”
The viral core is one of the factors common to all infectious poxvirus forms. “Previous experiments in virology, biochemistry, and genetics suggested several core protein candidates for poxviruses, but there were no experimentally-derived structures available,” says ISTA PhD student Julia Datler, one of the co-first authors of the study. Thus, the team started by computationally predicting models of the main core protein candidates, using the now-famous AI-based molecular modeling tool AlphaFold. In parallel, Datler was setting the project’s biochemical and structural foundations by drawing on her background in virology and the Schur group’s main expertise: cryogenic electron microscopy, or cryo-EM for short. “We integrated many of the most advanced cryo-EM techniques available today with AlphaFold molecular modeling. This gave us, for the first time, a detailed overall view of the poxviral core–the ‘safe’ or ‘bioreactor’ inside the virus that encloses the viral genome and releases it in infected cells,” says Schur. “It was a bit of a gamble, but we eventually managed to find the right mix of techniques to examine this complex question,” says postdoc Jesse Hansen, the study’s co-first author whose expertise in various structural biology techniques and image processing methods was pivotal for the project.
A global 3D view of the poxvirus
The ISTA researchers examined “live” Vaccinia virus mature virions and purified poxviral cores under every possible angle–quite literally. “We combined the ‘classic’ single-particle cryo-EM, cryo-electron tomography, subtomogram averaging, and AlphaFold analysis to gain an overall view of the poxviral core,” says Datler. With cryo-electron tomography, researchers can reconstitute 3D volumes of a biological sample as large as an entire virus by acquiring images while gradually tilting the sample. “It’s like doing a CT scan of the virus,” says Hansen. “Cryo-electron tomography, our lab’s ‘specialty,’ allowed us to gain nanometer-level resolutions of the whole virus, its core, and interior,” says Schur. In addition, the researchers could fit the AlphaFold models into the observed shapes like a puzzle and identify molecules that make up the poxviral core. Among these, the core protein candidate A10 stood out as one of the major components. “We found that A10 defines key structural elements of the core of poxviruses,” says Datler. Schur adds, “These findings are a great resource to interpret bits of structural and virological data generated over the last decades.”
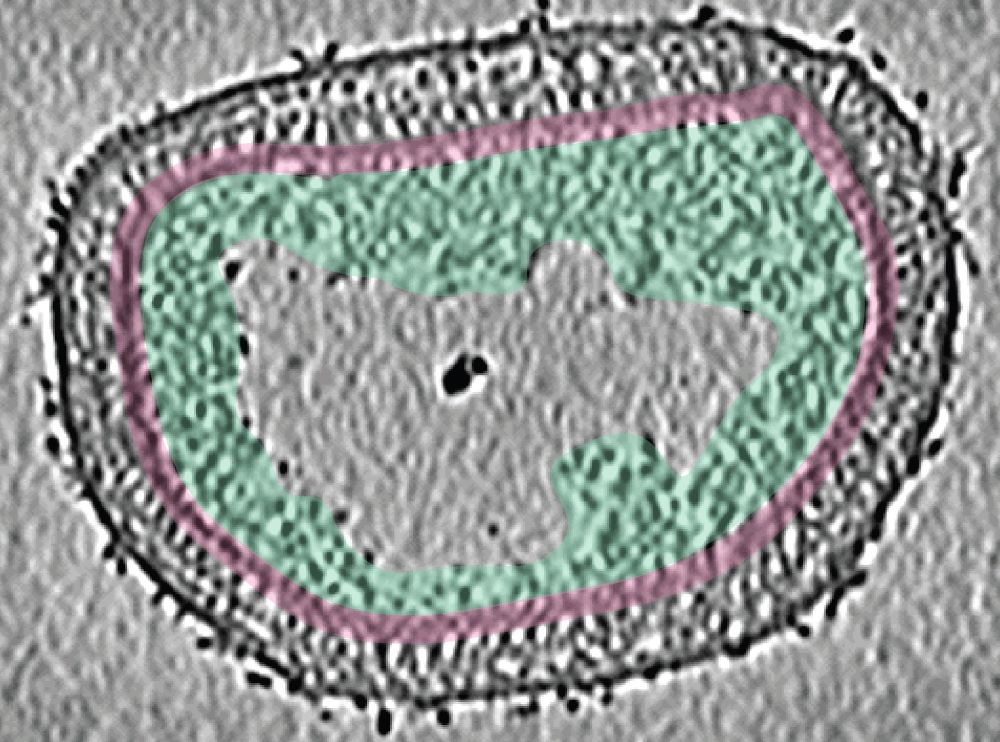
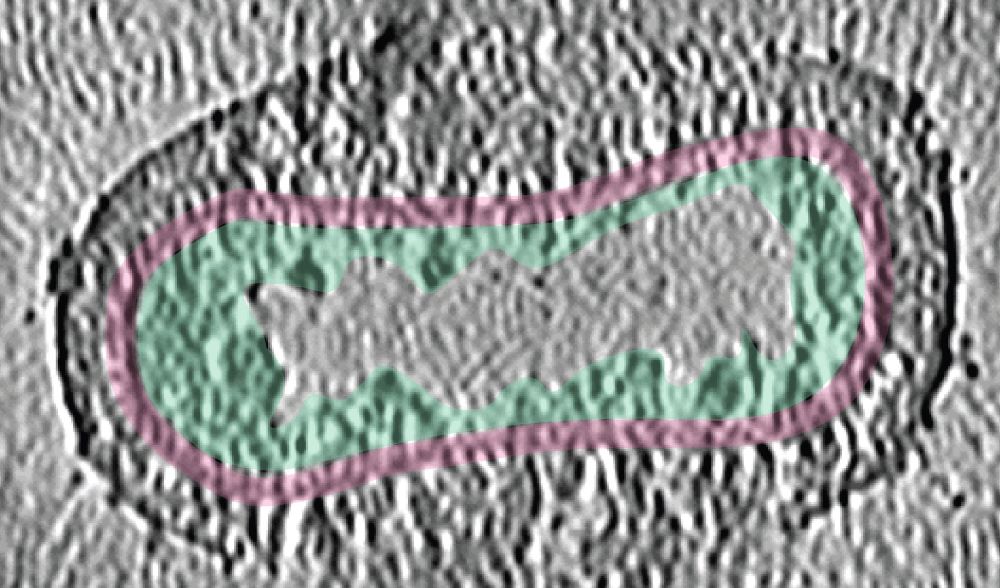
A rugged path to uncovering poxviral cores
The path to these findings was all but straightforward. “We needed to find our own way from the start,” says Datler. Leveraging her expertise in biochemistry, virology, and structural biology, Datler isolated, propagated, and purified samples of Vaccinia virus and established the protocols to purify the complete viral core, all while optimizing these samples for structural studies. “Structurally, it was extremely hard to study these virus cores. But luckily, our perseverance and optimism paid off,” says Hansen.
The ISTA researchers are convinced that their findings could provide a knowledge platform for future therapeutics that seek to target poxviral cores. “For example, one could think of drugs that prevent the core from assembling – or even disassembling and releasing the viral DNA during infection. Ultimately, fundamental virus research, as done here, allows us to be better prepared against possible future viral outbreaks,” concludes Schur.

All authors of this work are affiliated with the Institute of Science and Technology Austria (ISTA). The work is a collaboration between members of the Schur group (Julia Datler, Jesse M. Hansen, Andreas Thader, Lukas W. Bauer, Florian K. M. Schur), the Scientific Computing Unit (Alois Schlögl), and the Electron Microscopy Facility (Victor-Valentin Hodirnau).
Publication:
Datler J, Hansen JM, et al. 2024. Multi-modal cryo-EM reveals trimers of protein A10 to form the palisade layer in poxvirus cores. Nature Structural & Molecular Biology. DOI: 10.1038/s41594-023-01201-6
Funding information:
This project was supported by funding from the Chan Zuckerberg Initiative grant DAF2021-234754 and grant DOI: 10.37921/812628ebpcwg as well as the Austrian Science Fund (FWF) grant P31445.



